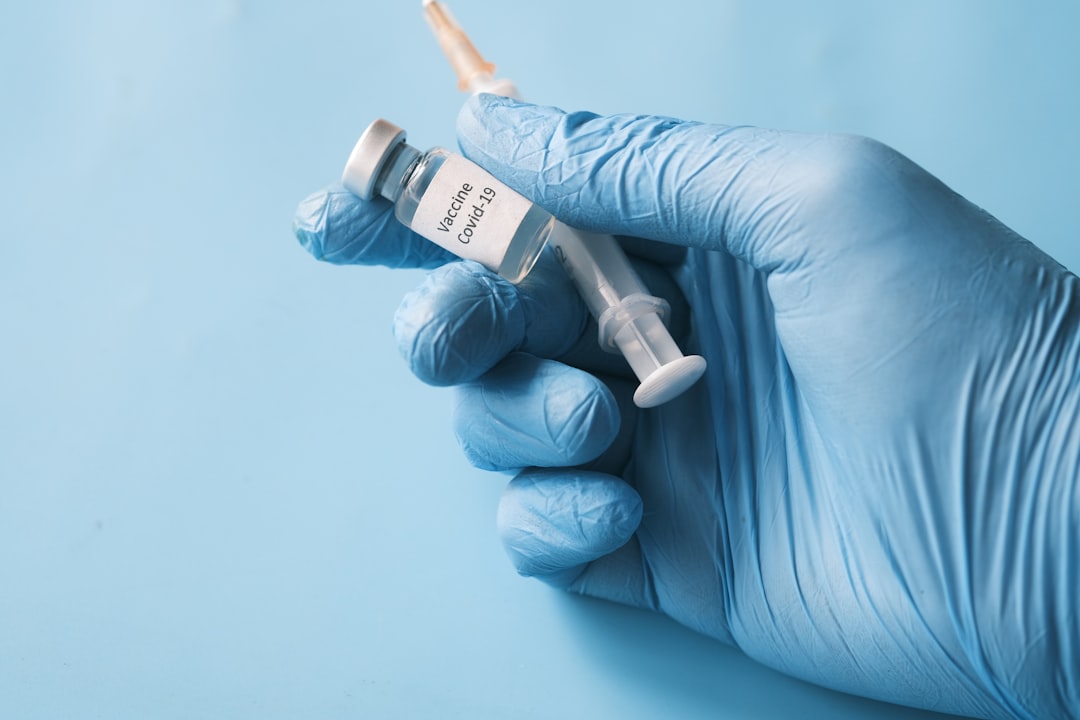
FDA Grants Conditional Approval to Novavax’s Protein-Based COVID Vaccine
Limited Authorization and Clinical Context
The FDA has approved Novavax’s COVID-19 vaccine—but only for adults over 65 and individuals aged 12+ with high-risk conditions. This targeted authorization reflects:
-
Traditional platform: A protein-based formulation, in contrast to mRNA shots.
-
Regulatory delay: Approval missed the April 1 target, fueling uncertainty.
-
Policy shift: Aligns with expert panel discussions on narrowing annual-shot recommendations.
Novavax’s Chief Corporate Affairs Officer Silvia Taylor emphasized that focusing on those most at risk mirrors evolving CDC guidance, suggesting broader policy moves toward more selective immunization.
Market Reaction and Share Dynamics
Novavax shares jumped on the approval news but remain volatile amid mixed efficacy perceptions and high-profile skepticism from public figures. Key drivers include:
-
Investor sentiment: Approval restores confidence but limited scope tempers upside.
-
Comparative efficacy: Ongoing head-to-head studies against mRNA vaccines will influence uptake.
-
Next-season filings: Novavax must update strain composition to compete in the upcoming immunization cycle.
Financial Health and Analyst Metrics
To assess Novavax’s capacity to fund new clinical trials and manufacturing scale-up, review its latest liquidity and leverage figures via the Balance Sheet Statements API. Meanwhile, tracking shifts in consensus outlooks and target revisions can be done through the Price Target Summary API.